Developing a recombinant gut antigen and evaluating its impact on adult sea lice: ImmuLice
The focus of this project was to explore the potential for a vaccine targeting the adult, blood-feeding stages of L. salmonis, using a recombinant antigen known as Cathepsin L (CatL).
Project team
Partners: AquaTreck, University of Stirling’s Institute of Aquaculture, Moredun Research Institute, Moredun Scientific
Authors: Dr Sean Monaghan, Prof. James Bron, Dr Michael McGowan, Dr David Bassett, Dr Panos Christofilogiannis, Dr Ansgar Stratmann, Dr Kim Thompson, Dr Bill Roy
Impact
The ImmuLice project has made several lasting contributions to the field of aquaculture health management. It produced a stable and scalable recombinant antigen platform, created a new adult louse challenge model for vaccine testing, and established transcriptomic and immunological analytical pipelines for evaluating host–parasite interactions.
£76,000
Total value
Case study
Download PDF
BACKGROUND
Sea lice (Lepeophtheirus salmonis) remain a challenge in Atlantic salmon farming. These parasites cost the Scottish aquaculture sector over £60 million annually, with a cost of more than £700 million globally. A range of pharmaceutical and biological treatments are available, although the potential for growing parasite resistance can reduce the effectiveness of traditional control methods.
Developing an effective vaccine offers a sustainable, environmentally friendly alternative that could significantly reduce the need for pharmaceutical treatments, improve fish welfare, and enhance production capacity. To date, however, no commercially available vaccine has proven effective against sea lice.
The project outlined in this case study, ImmuLice, built upon a previous SAIC-funded sea lice vaccine study that demonstrated immune stimulation in salmon against early larval lice stages. ImmuLice brought together AquaTreck, the University of Stirling’s Institute of Aquaculture, Moredun Research Institute and Moredun Scientific, and ran for 15 months with support from SAIC. Its focus was to explore the potential for a vaccine targeting the adult, blood-feeding stages of L. salmonis, using a recombinant antigen known as Cathepsin L (CatL). Unlike larval lice, which feed mainly on surface mucus and epithelial tissue, adult lice consume blood and may be more directly affected by systemic immune factors such as circulating antibodies.
AIMS
The project aimed to:
- Develop and test an injectable vaccine targeting the adult stages of sea lice using a recombinant gut protein antigen, Cathepsin L (CatL);
- Assess the vaccine’s effect on lice attachment, fecundity, and physiology;
- Establish a reliable adult sea lice challenge model for future vaccine efficacy testing;
- Explore host immune responses and parasite transcriptomic changes resulting from vaccination.
Methodology and overview
The project was structured around three main work packages. The first (WP1) aimed to establish reliable production of the recombinant CatL antigen, a gut protein of the sea louse that had previously shown promise in stimulating an immune response. Using the Pichia pastoris yeast expression system, AquaTreck optimised the expression and purification of the CatL protein. The team tested different temperature profiles and feed mixtures of methanol and glycerol to stabilise production and improve yield.
The second work package (WP2) focused on refining and enhancing production methods to make vaccine development viable on a larger scale. Further optimisation of fermentation parameters and purification processes produced a stable formulation that could be stored without loss of antigenicity. The resulting vaccine preparation contained highly concentrated CatL protein and was emulsified with a commercial adjuvant for intraperitoneal (IP) injection.
The third and final work package (WP3) evaluated the vaccine’s efficacy using an adult sea lice challenge model developed specifically for this study. Atlantic salmon smolts were divided into vaccinated and control groups and maintained in replicate tanks. Each vaccinated fish received a single IP injection of the recombinant CatL vaccine, while control fish received adjuvant in buffer solution. After an appropriate immunisation period, both groups were exposed to adult male and female sea lice under controlled cohabitation conditions designed to mimic realistic infection pressures. Blood samples and tissues were collected from vaccinated and control Atlantic salmon at different time points post-vaccination for assessing antibody response using ELISA, as well as measuring immune responses in fin and spleen, respectively, with qPCR.
RESULTS
The project’s results were both technically and scientifically significant. The Pichia pastoris system proved capable of producing high yields of stable recombinant CatL antigen suitable for vaccine use, demonstrating a scalable platform for future antigen production. The recombinant antigen was purified to over 90% and confirmed by western blot analysis to retain its structure and immunogenicity. This stable and scalable expression platform was essential for generating enough antigen for vaccine formulation and testing.
Through the work in WP2, the team created a reproducible and high-quality vaccine candidate for testing in Atlantic salmon. The vaccinated fish in WP3 showed measurable immune responses, including antibody binding to the recombinant antigen, but no significant reduction in lice numbers or egg viability was observed compared with control fish. Gene expression analysis by qPCR also found no clear differences in antibody-related markers such as IgM or MHC II between groups.
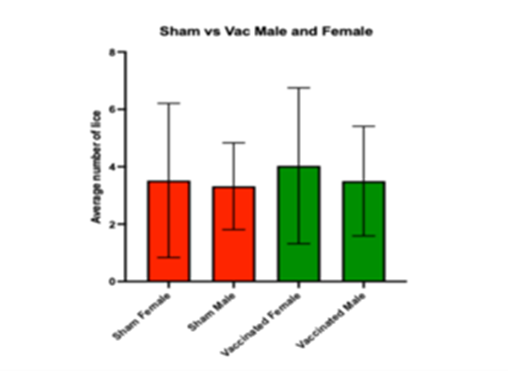
Figure 1. Adult male and female L. salmonis counts post-vaccination on Atlantic salmon challenged with recombinant CatL2. Each bar indicates an independent tank of challenged fish. N=6.
However, when researchers examined the lice themselves, important insights emerged. RNA sequencing of adult female lice collected from vaccinated fish revealed distinct transcriptomic differences from those feeding on unvaccinated fish. These lice showed greater variability in gene expression, suggesting that the vaccine had altered their physiological state. Notably, genes linked to stress and cellular repair – including heat shock proteins and cortactin-binding protein 2 – were upregulated, while several uncharacterised genes were downregulated. Although these molecular changes did not translate to fewer lice on the fish, they indicated that host vaccination could still influence parasite biology in subtle but meaningful ways.
Parallel analyses of salmon skin samples revealed how the host’s immune system responded locally to louse attachment. Expression of wound-healing genes (MMP9 and MMP13) was highest directly under the lice’s feeding sites and declined with distance from the wounds. While differences between vaccinated and control fish were not statistically significant, there was a trend suggesting that an elevated vaccine dose may have moderated these responses. Histological examination of skin sections confirmed that the adult lice’s feeding apparatus caused distinct tissue damage and cellular infiltration, providing valuable material for future study.
Together, these findings showed that while the vaccine did not yet prevent lice infestation, it did induce physiological stress in feeding lice and helped establish analytical methods for understanding vaccine effects beyond simple parasite counts. The adult lice challenge model developed through this work now provides a robust foundation for testing future vaccine candidates targeting the hematophagous stages of L. salmonis.
IMPACT
The ImmuLice project has made several lasting contributions to the field of aquaculture health management. It produced a stable and scalable recombinant antigen platform, created a new adult louse challenge model for vaccine testing, and established transcriptomic and immunological analytical pipelines for evaluating host–parasite interactions. It also strengthened collaborations among key Scottish research institutions and industry partners, while providing training opportunities for postgraduate students and early-career researchers in vaccinology, immunology, and RNA sequencing analysis.
Although a protective vaccine outcome was not achieved at this stage, the work demonstrated that host vaccination can influence parasite gene expression, providing a new avenue for investigating how immune responses affect sea lice biology. The new challenge model was a major achievement in itself, allowing the team to directly assess vaccine effects on adult lice attachment, fecundity, and physiology – something not previously possible using larval challenge models. The partners plan to build on this foundation by developing multi-antigen formulations, supported by new funding proposals.
In summary, ImmuLice advanced both the technical and conceptual understanding of how vaccination may be used to control sea lice. It has provided essential tools, data, and collaborative momentum for the next generation of vaccine research, progress that moves the salmon farming sector closer to an increasingly sustainable, welfare-focused approach to parasite management.