Artificial Intelligence approaches to improve diagnosis of gill disease in Atlantic salmon (AIGD)
Successful proof-of-concept application of Artificial Intelligence (AI) technologies for improved gill health diagnostics through automated classification of histopathological markers of gill disease in farmed Atlantic salmon.
Impact
This project brought together the expertise required to address the bottlenecks associated with the traditional and labour-intensive methods of gill health diagnostics. This proof-of-concept work clearly demonstrates that AI technologies could potentially be used to improve gill diagnostics in sea-farmed Atlantic salmon.
£79,863
Total value
Partners
-
University of Aberdeen
-
Scottish Fish Immunology Research Centre
-
Vertebrate Antibodies
-
Scottish Sea Farms
-
BioMar
-
Marine Scotland Science
Project leads
Professor Sam Martin, Dr Ela Król, Dr Alex Douglas, Dr Ayham Alnabulsi, Dr Ralph Bickerdike, Dr Victoria Valdenegro, and Dr Patricia Noguera
Case study
Download full Case Study PDF
BACKGROUND
The gill of teleost fish is a multifunctional organ involved in many physiological processes, such as gas exchange, osmotic and ionic regulation, acid-base balance, and excretion of nitrogenous waste, as well as playing a key role as primary mucosal defence tissue against pathogens. The prevalence of gill damage and gill diseases among farmed Atlantic salmon has increased in recent years due to extreme weather events, warming waters, and emerging pathogenic organisms, leading to substantial losses for the aquaculture sector, both in Scotland and globally.
Monitoring gill health along with the diagnosis of gill pathologies, such as proliferative gill disease (PGD), proliferative gill inflammation (PGI) and complex gill disorder (CGD), is commonly based on histopathological analysis and the ‘scoring’ of gills by fish pathologists and on-farm health personnel. This manual analysis, however, is labour intensive and may be open to subjectivity or bias, therefore acting as a bottleneck to delivering timely results. Improving the tools available for gill health diagnostics is essential for the early detection of gill disease, for developing strategies to monitor gill health and for fine-tuning salmon husbandry practices to address the challenging conditions of a rapidly changing marine environment.
Artificial Intelligence (AI) along with its subdisciplines of Machine Learning (ML) and Deep Learning (DL) are emerging as key technologies in medicine and pathology, opening avenues for faster, more reproducible, and more precise diagnosis through computerised image analysis. Several AI technologies have recently produced ground-breaking results in image classification and interpretation, however, the application of these technologies to gill health diagnostics had yet to be explored.
This project set out to evaluate the applicability of AI technologies to monitor gill health in Atlantic salmon, bringing together leading experts in fish health from the Scottish Fish Immunology Research Centre at University of Aberdeen with software and image analysis specialists at Vertebrate Antibodies Ltd., along with sector partners Scottish Sea Farms Ltd, BioMar Ltd, and Marine Scotland Science. Using an existing dataset of gill histopathology images generated by two previous SAIC-funded projects (Clinical nutrition and the treatment of Atlantic salmon gill diseases (NAGD) and Spatial and Temporal Drivers of Gill Pathology in Atlantic Salmon (STGP)), project partners proposed to develop a prototype of automatic classification of histopathological markers of multifactorial gill disease, providing a roadmap for commercial product development. Such software would be applicable for high-throughput gill sample screening (research or diagnostic) and early detection of gill pathologies, contributing to rapid response and implementation of appropriate interventions and treatments. Moreover, the resultant computer-aided diagnostic tool for faster, cheaper, and more precise gill image analysis would unlock additional capacity to maintain healthy population of farmed salmon, thereby benefiting the industry, consumers, fish welfare and the environment.
AIMS
The overarching goal of this proof-of-concept study was to assess the application of AI technology for monitoring gill health in Atlantic salmon using the existing dataset of gill histopathology images, scored by an experienced fish pathologist. Furthermore, the project aimed to develop a prototype of automated classification of histopathological markers of gill disease.
In this study, project objectives were categorised into distinct work packages focusing on the identification of gill health parameters for diagnostics, assessment and comparison of leading AI technologies, and software development to address the following main aims:
- To develop a set of optimised histopathological parameters for diagnosis and assessment of gill disease.
- To identify the AI software best suited for diagnosis and assessment of gill disease.
- To produce and launch a demo version of the Gill Image Analysis Software (GillsPipe).

Identification of the leading histopathological parameters of gill disease
For the purposes of assessing gill disease, the fish pathologist manually analysed and scored gill histopathology images using well established protocols (Król et al. 2020). The scoring system (adapted and modified from Mitchell et al., 2012) consisted of 4 index and 11 ancillary criteria, scored from 0 to 3:
- Score 0: No pathological changes.
- Score 1: (Mild) < 10% of gill tissue impacted.
- Score 2: (Moderate) 10–50% of gill tissue impacted.
- Score 3: (Severe) > 50% of gill tissue impacted.
The index criteria included 1) lamellar hyperplasia, 2) lamellar fusion, 3) lamellar oedema and 4) cellular anomalies, while the ancillary criteria referred to the presence of 1) inflammation, 2) eosinophilic granular cells, 3) chloride cells, 4) circulatory disturbances, 5) interlamellar blood, 6) cellular hypertrophy, 7) Epitheliocystis-like bacteria, 8) Tenacibaculum-like bacteria, 9) other bacteria, 10) protist Neoparamoeba-like parasites, and 11) other parasites or agents.
Many of these parameters were redundant in terms of diagnostics and therefore the first step in this work was to separate the leading parameters (i.e., the best suited for the diagnosis of gill disease) from the redundant criteria, using statistical and machine learning approaches. This would allow only the most defining parameters to be taken forward for training the AI model in development of the automated scoring system.
The analysis and identification of the leading histopathological parameters of gill disease was carried out using the existing dataset of gill histopathology images, which were scored by the fish pathologist.
The separation of the leading and redundant parameters was based on the statistical (non-metric multidimensional scaling and eigenvectors) and AI (machine learning) approaches with results critically evaluated by the fish pathologist.
The comparison between computer and manually selected parameters identified ‘lamellar hyperplasia’, ‘lamellar fusion’, and ‘vascular anomalies’ as the three leading histopathological markers of gill disease. These three parameters were taken forward for the next phase of the project to train the AI model when developing the automated scoring system of gill disease.
Development of the automated scoring system of gill disease
This stage of the project was broken down into three sequential steps to first identify, then train and test the most appropriate AI model and subsequent software.
Selection of the model
As part of this, three different machine learning models were evaluated to establish which AI approach was the most suitable for identification and classification of the leading histopathological parameters of gill disease (lamellar hyperplasia, lamellar fusion, and vascular anomalies), based on a small selection of gill images annotated by the fish pathologist. The three AI models tested were:
1) Object Detection Approach (YOLOv5)
2) Semantic Segmentation Approach
3) Object Recognition (Vision Transformer (VT))
Comparison of performance of the three different machine learning models led project partners to focus on the Vision Transformer (VT) as the best suited for diagnosis and assessment of gill disease. The VT model is a type of deep learning model that is trained to understand the context and relationships within an image. As part of this comparison, researchers fine-tuned and pre-trained VT model on the dataset of gill images using the annotated labels for the three leading histopathological parameters. This was followed by the analysis of the performance of the VT by comparing its predicted labels to the pathologists’ annotations. Selection of this model was predominantly based on the flexibility of the model that is needed when dealing with the large number of features in the gill tissue, and capacity of the model to be re-trained and expand.
Training the model
The next step of this work was to fully train the VT model to recognise the diagnostic parameters (lamellar hyperplasia, lamellar fusion, and vascular anomalies) using a larger selection of gill images annotated by the fish pathologist.
The training process was performed using a coordinated computer program and consisted of different steps, starting with generating small patches of the image usually of a fixed size. Each patch was then flattened into a one-dimensional array of pixels with added positional embeddings creating a sequence of pixel values that served as an input for the VT model. To account for the spatial relationships between pixels, positional embeddings were added to the input sequence. This allowed the model to understand the position of each pixel within the patch. The model was then re-trained with labelled images to generate the most accurate classification. This resulted in the development and execution of a series of code fragments to filter and structure the data.
The accuracy for the resulting trained model was 91%, 98% and 97% for lamellar hyperplasia, lamellar fusion, and vascular anomalies, respectively.
Testing the model
Finally, the trained model was tested using a new dataset of gill images that were not part of the original training or evaluation processes. The testing was performed using a Python script and the resulting output for each tested image was a ‘tiles-eval.txt file’ (tiles geolocation with their corresponding raw data indicator as positive (red) or negative (green)) and a map (image file with masks representing the predicted anomalies as in Figure 1). The Python script also generated a ‘stats.txt file’, containing a numerical report of the AI image analysis.

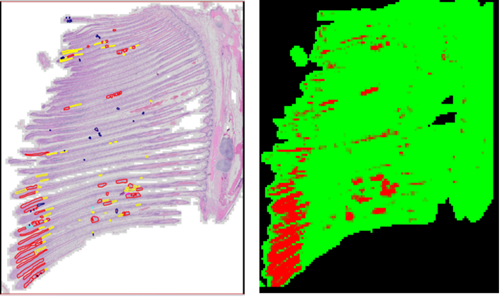
Figure 1: The output of AI software with a map image file of positive lamellar fusion (red) and negative background (green). Left: Original slide with annotations. Right: Map generated by the software based on the slide without the annotations.
Outcomes
Overall, the VT model demonstrated a strong efficacy in identification and classification of histopathological markers of gill diseases from digital gill images, as tested for lamellar hyperplasia, lamellar fusion, and vascular anomalies. Importantly, the VT software generated two different types of output: 1) image representation of the predicted anomalies that can be easily compared with the manual annotation by the fish pathologist, and 2) a numerical representation of factors that show the prevalence of anomaly in each gill image file. As a result, the VT model was used as a base for development of a demo version of the Gill Image Analysis Software (GillsPipe).
The GillsPipe prototype
The demo version of GillsPipe was generated by an assembly of all the best suited scripts and approaches previously identified. The idea behind producing the demo package was to test the system independently and provide feedback that could be used in future work to optimise the software performance to prepare for commercialisation.
The GillsPipe software was inspected by project partners using a further 38 unannotated gill images originated from 17 fish. The software outputs for lamellar hyperplasia, lamellar fusion, and vascular anomalies were compared to the scores provided by the fish pathologist.
Even while only using a relatively small number of images to train the system, preliminary results were encouraging. Specifically, the trained models were able to detect gill anomalies with high accuracy and the AI output for lamellar hyperplasia, lamellar fusion, and vascular anomalies was positively correlated with the manual scores generated by the fish pathologist.
Project partners agreed that generating the demo version of GillsPipe was a highly successful exercise, providing the proof-of-concept evidence that AI technologies could be effectively employed for automated classification of histopathological markers of gill disease.
The demo version of GillsPipe is currently available on request from project partners at Aberdeen University with all essential requirements and codes that are necessary to run the software independently from the user’s environment. However, the current version of GillsPipe is a developer version and requires some experience and knowledge in programming and command language to fully utilise the software. To fully expose GillsPipe for robust testing and commercialisation, more work is required to automate the pipeline to a more user-friendly version, develop the software interface, and avoid the need for any code editing by the user.
Further testing and formal commercialisation of the GillsPipe software was not possible within the remit of this project due to time and budget constraints. Furthermore, the capacity of the computers was a limiting factor within this project, only allowing a maximum of ~38 gill images to be processed. However, to successfully retrain the existing models for lamellar hyperplasia, lamellar fusion, and vascular anomalies (or for other gill histopathological markers), more than 200 gill images would need to be used, which is a standard approach in medical applications of the AI technologies.
IMPACTS
Gill health is central to overall fish physiology and performance. At the farm level, understanding various gill disorders, the potential risk factors and the disease-specific diagnoses are important for the development of best practice approaches for mitigation and for improvement of the overall health of farmed fish.
This project brought together the expertise required to address the bottlenecks associated with the traditional and labour-intensive methods of gill health diagnostics. This proof-of-concept work clearly demonstrates that AI technologies could potentially be used to improve gill diagnostics in sea-farmed Atlantic salmon.
The prototype software, GillsPipe, developed as part of this work allowed for the automated classification of histopathological markers of gill disease and generated output that was in a good agreement with the scores from the fish pathologist. Although the prototype version of the software is available at request from project partners, more work is required to fully develop and utilise the benefits of the AI approach for further commercialisation. A fully developed AI software for gill diagnostics could modernise and advance the aquaculture diagnostics and research services, by incorporating a tailored AI tool that could accelerate the screening for gill pathologies, ultimately improving health of farmed fish. Furthermore, an improved diagnostic service would contribute to more informed decision making for fish health professionals, with the anticipated impact of improved welfare and survival of farmed fish leading to greater productivity for the Scottish aquaculture sector.
This work created a unique opportunity for the fish pathologist to work closely with the AI engineers, and similarly, introduced the AI experts to the complexity of multifactorial gill disease. Furthermore, this has demonstrated that through collaboration and combining interdisciplinary approaches, we could generate better solutions to the problem of fish health in the aquaculture setting.
Additional information
References
Król E, Noguera P, Shaw S, Costelloe E, Gajardo K, Valdenegro V, Bickerdike R, Douglas A, Martin SAM. Integration of Transcriptome, Gross Morphology and Histopathology in the Gill of Sea Farmed Atlantic Salmon (Salmo salar): Lessons From Multi-Site Sampling. Front Genet. 2020 Jun 19;11:610. doi: 10.3389/fgene.2020.00610. PMID: 32636874; PMCID: PMC7316992.
Mitchell, S. O., Baxter, E. J., Holland, C., and Rodger, H. D. (2012). Development of a novel histopathological gill scoring protocol for assessment of gill health during a longitudinal study in marine-farmed Atlantic salmon (Salmo salar). Aquacult. Int. 20, 813–825. doi: 10.1007/s10499-012-9504-x